Dialysis Disequilibrium Syndrome

Clinical Scenario:
You respond as an ALS ambulance to a local dialysis center for a middle-aged male with altered mental status. Upon arrival, you find a conscious male being gently held in his dialysis chair by staff members. They report the patient was partially through his dialysis treatment when he developed acute delirium.
| Medical History | Medications | Allergies |
| ESRD | Coumadin, Epogen | PCN |
| MI | ASA 81 mg | Sulfa drugs |
| HTN | Lisinopril | |
| CVA | Warfarin | |
| NIDDM | Metformin 500 mg |
Vital signs are as follows: BP 172/100, HR 110 NSR, RR 18, SpO2 94% RA, FSBG 107 mg/dL, EtCO2 37 mmHg
No apparent focal deficits are present—no obvious signs of physical injuries. The patient is holding his head, which is perceived as an acute headache. The patient does not endorse dizziness, vision changes, or nausea/vomiting. Further, the patient has no prominent gait disturbances, coordination abnormalities, or speech changes. The patient is afebrile with no illness symptoms. The patient is transported to the local hospital for a complete neurological evaluation. During transport, the patient becomes combative, which is not resolved with therapeutic communication. He requires IV midazolam during transport to minimize the level of agitation.
What is going on with this patient? What conditions are included in your differential diagnosis?
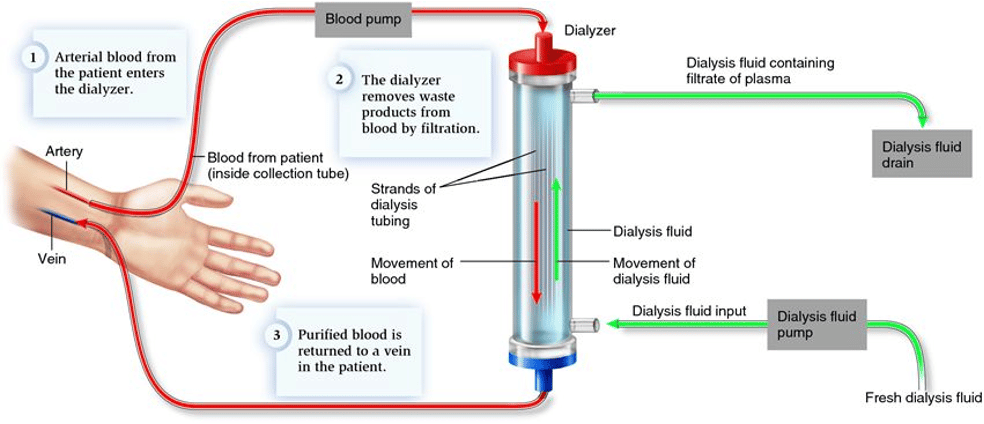
Case Review:
This patient presents with a rare and interesting complication of hemodialysis called dialysis disequilibrium syndrome (DDS). DDS leads to various neurological signs/symptoms, including headache, mental status changes, agitation, nausea/vomiting, hypertension, visual changes, asterixis, seizures, coma, and death (1,2,3). The true prevalence of DDS is unknown since only moderate-severe symptoms are commonly documented. The purpose of this article is to review DDS and ensure familiarization with other standard dialysis complications.
As a review, patients with severe renal disease have an impaired ability to filter toxins and natural waste products (1). One of these waste products, called urea, tends to build up in between dialysis appointments. Interestingly, both high urea levels and the rapid removal of urea during hemodialysis can cause neurological symptoms (1). The exact physiological mechanism of this complication is not very well understood (2). However, a few theories exist that contribute to cerebral edema and increased intracranial pressure. The first theory involves a rapid reduction in blood urea compared to brain urea levels, which creates an osmotic gradient; as a result, water moves into the brain through osmosis (3). A competing theory relies on chronic hyperosmolality in CKD patients due to hyperglycemia or hypernatremia. The body produces osmoles, preventing an osmotic gradient and severe fluid shifts between plasma and cerebral cells. However, blood glucose and sodium levels deplete during hemodialysis, leading to a strong osmotic gradient that causes water to flow into the brain (3). Lastly, renal disease patients have chronic metabolic acidosis and subsequent hyperventilation. During dialysis, acidosis is corrected, causing a reduction in the ventilatory rate and a subsequent rise in CO2 levels. The CO2 diffuses into the brain and CSF, increasing osmolality and causing cerebral edema (3). To review, patients with acidosis have a right shift on the oxyhemoglobin curve (4). During dialysis, alkalization causes a left shift, increasing hemoglobin affinity for oxygen (4). As a result, tissues experience less oxygen offloading, and cerebral hypoxia development can increase neurological symptoms (3).
Ultimately, the best treatment for this complication is prevention (2,3). Risk factors for developing DDS include age extremes, hyperosmolality (severe uremia, hypernatremia, & hyperglycemia), CNS disorders, metabolic acidosis, severely elevated pre-dialysis BUN, previous cerebral insult, and the existence of conditions that cause cerebral edema (3). Patients with risk factors for DDS or earlier instances of this condition may benefit from dialysis treatment setting changes. For example, slower dialysis treatments (longer treatment time, slower blood flow rate, and smaller dialyzers) have been shown to reduce the prevalence of DDS (5). In addition, performing dialysis with a high sodium dialysate allows osmolality to remain in a consistent range (2,3). Lastly, adding an osmotically active substance such as sodium, mannitol, or glucose can reduce the development of an osmotic gradient, limiting pathological water shifts and the subsequent development of cerebral edema (2,3). Therefore, during these calls, an essential part of the history gathering is to ask about current dialysis settings and any recent changes. In this case, EMS was called after the fact and had to begin treatment after prevention had failed. While interesting, it is essential to remember that this phenomenon is a diagnosis of exclusion (2). For pre-hospital providers, performing a thorough assessment and developing a differential diagnosis is crucial. Treatment for this condition is primarily supportive. Patients that have a reduced level of consciousness may require airway management. Further, EMS clinicians should maintain a high index of suspicion for seizure development. Usually, these patients will gradually improve after the termination of dialysis. However, in some cases, hypertonic saline and mannitol medications increase plasma osmolality and reduce cerebral edema (3).
|
Differential Diagnosis (2) |
Assessment Tools |
|
Hypoglycemia |
FSBG < 70 mg/dL (6)
S/S: pale or diaphoretic skin conditions, tremors, mental status changes, fatigue, Confusion, slurred speech, ataxia, coma, seizures |
|
Diabetic Ketoacidosis |
FSBG > 250 mg/dL (7) Arterial pH < 7.3 (ABG); Anion Gap > 10 mEq/L Serum bicarbonate < 18 mEq/L S/S (8): polyuria, polydipsia, polyphagia, headache, nausea/vomiting, Kussmaul’s respirations, AMS, dry skin conditions, abdominal pain |
|
Intracranial hemorrhage |
Neurological exam + CT (9) S/S: headache, nausea/vomiting, vision changes, AMS, neurological deficits, altered level of consciousness, seizures, coma Cushing’s triad: ↑ BP, ↓HR, irregular respirations |
|
CVA/TIA |
Neurological exam, non-contrast CT, CTA, perfusion imaging CBC, BMP, Coagulation Profile, cardiac markers, hemoglobin A1C, TTE, cardiac telemetry, & neck vessel imaging – are utilized to determine etiology (10)
S/S: headache, confusion, speech changes, vision changes, neurological deficits, ataxia, coordination changes |
|
Meningitis |
CSF evaluation (lumbar puncture), CBC S/S: fever, headache, photophobia, nuchal rigidity, neck pain, AMS, nausea (11)
|
|
Encephalopathy |
Numerous causes to evaluate – CBC, liver function tests, ammonia levels, glucose levels, lactate levels, renal function test, blood cultures, virology tests, ABGs, neuroimaging, EEG (12) S/S: AMS, cognitive decline, personality changes, myoclonus, nystagmus, tremors, loss of ability to speak or talk, seizures (13) |
|
Hyponatremia |
Serum sodium < 135 mEq/L (14) Urine osmolality – depends on the type of hyponatremia.
S/S: weakness, nausea/vomiting, headache, confusion, sleepiness, seizures, cardiorespiratory distress |
Hemodialysis has become increasingly safe over time, but certain complications do remain. Symptoms such as headache, dizziness, and muscle cramps are common after dialysis and are typically attributed to aggressive ultrafiltration or a greater degree of solute removal (15). Intradialytic hypotension is another common problem associated with dialysis treatment. A widely accepted definition includes a decrease in systolic blood pressure by 20 mmHg or a reduction in MAP by 10 mmHg (17). Numerous causes of intradialytic hypotension exist, but the primary reasons include interdialytic fluid weight gain, ultrafiltration, reduced osmolality, autonomic dysfunction, and reduced cardiac reserve (17). It is crucial to consider other complications, including air embolism, internal hemorrhage, dialyzer reaction, and various cardiovascular conditions (MI, ischemia, pericardial tamponade, and pericardial effusion). Intradialytic hypotension increases fluid removal so that intravascular volume cannot be replenished or the body’s intrinsic mechanisms cannot compensate (15,17). As a reminder, reduced blood volume causes less preload, stroke volume, and cardiac output. The body’s physiological response to reduced blood volume is to increase HR (CO = SV x HR). It is essential to remember that certain medications (alpha-1 blockers, beta-blockers, etc.) may impact a patient’s ability to compensate for reduced blood volume. Therefore, these patients are more likely to be symptomatic and require emergency treatment. Management of this condition includes stopping dialysis, placing the patient in a supine position if feasible, and providing small boluses of IV isotonic fluids. If hypotension leads to impaired renal perfusion, episodes of intradialytic hypotension may lead to worsening renal function.
Interestingly, another complication associated with dialysis is hypertension. Patients at risk for this condition include those with pre-existing hypertension, interdialytic water weight gain, and an overactive RAAS system as fluid removal occurs (16). It may take up to two weeks for their blood pressure to normalize after treatment (16). Common causes of intradialytic hypertension include volume overload, use of a high sodium dialytic agent, dialytic removal of anti-hypertensive medications, and SNS response to reduced blood volume (16). While non-dialyzable anti-hypertensive may help lower intradialytic hypertension, the literature suggests that volume control may be the best method (18).
Cardiac dysrhythmias are another common complication associated with hemodialysis. Adult patients are more likely to have co-existing cardiovascular conditions, including left ventricular hypertrophy (secondary to chronic hypertension/anemia), cardiomyopathy, and uremic pericarditis (15,16). In addition, episodes of intradialytic hypotension may reduce coronary artery perfusion, leading to myocardial ischemia and subsequent dysrhythmias. Besides these risk factors, patients undergoing dialysis have rapid electrolyte changes in potassium and calcium, both of which have profound cardiovascular effects (15,16). Possible preventative measures include close monitoring of electrolytes during dialysis and using a bicarbonate dialysate. Patients receiving dialysis are at higher risk of sudden cardiac arrest due to various factors, including myocardial ischemia and hyperkalemia (16). While EMS providers are not expected to master dialysis machines, discussing the current settings or any mechanical errors with dialysis staff is essential. For example, problems such as an air embolism, inappropriate dialysate concentration, over-heated dialysate, and line disconnection may lead to sudden cardiac death due to dialysis machine errors (16).
While rare, acute hemolysis is a significant concern for patients receiving hemodialysis. The causes of this condition are vast and will not be discussed in this article for brevity. Patients with this condition develop erythrocyte damage, reducing the oxygen-carrying capacity of circulating blood. Another problem is the release of intracellular contents into the bloodstream. The presentation of this complication is relatively non-specific and includes abdominal pain, nausea/vomiting, chest pain, dyspnea, and back pain. Other findings that may be present consist of a port-wine appearance of blood in the venous line and a pink discoloration of plasma in centrifuged specimens (15,19). If undetected, hyperkalemia will occur, causing weakness, muscle cramps, ECG changes, and cardiac arrest (15). If detected, dialysis should immediately stop, and blood in the external circuit should not re-enter the patient’s bloodstream. Treatment includes close monitoring of bloodwork, hyperkalemia management, repeat dialysis for severe hyperkalemia, or blood transfusions for anemia (15,19).
Hemodialysis patients with underlying diabetes, peripheral vascular disease, or Raynaud’s syndrome are at higher risk of dialysis-associated steal syndrome (16). This is a rare complication of dialysis that causes reduced perfusion to the extremity distal to the AV fistula (16). EMS providers should look for the 6 P’s familiar in similar conditions such as an arterial occlusion (pain, pallor, poikilothermic, pulselessness, paresthesia, and paralysis). Other non-invasive assessment tools include reduced doppler perfusion and inconsistent plethysmography with lower amplitude (16). Treatment of this condition depends on the clinical severity, but it may be a surgical emergency if the above signs/symptoms exist.
Lastly, the AV fistula serves as a direct connection to the bloodstream. As a result, it can increase the risk of air embolisms, sepsis, and hemorrhage (16). Air embolisms have become increasingly rare due to dialysis machines detecting air in the circuit. However, it should remain a differential diagnosis in patients with presentations similar to PE, CVA, MI, etc. Additional complications of hemodialysis exist but are not discussed in this article.
Dialysis patients are usually very knowledgeable about their condition and can serve as excellent historians. In addition, dialysis facilities typically have adequate documentation of disorders, dialysis visits, and recent bloodwork. In preparation for managing dialysis patients, learning about these acute complications and how to treat them best is essential. In addition, understanding the associated lab work will help in pre-hospital and critical care transport settings.
|
Lab Value |
Importance |
|
Glomerular Filtration Rate (GFR) |
Defines the stage of renal disease (1) Stage 1: renal damage with normal GFR Stage 2: GFR 60 – 89 mL/min Stage 3A: GFR 45 – 59 mL/min Stage 3B: GFR 30 – 44 mL/min Stage 4: GFR 15 – 29 mL/min Stage 5: GFR < 15 mL/min |
|
CBC |
Anemia – low hemoglobin levels (20) Infection – abnormal WBC values |
|
BUN + Creatine |
Elevated BUN + creatine indicates renal damage (1) Normal BUN: 6 – 24 mg/dL Normal Creatinine: 0.6 – 1.3 mg/dL
The ratio of BUN and creatine can also be used to evaluate dehydration/hypovolemia. BUN: creatinine ratio of above 20:1 indicates dehydration/hypovolemia |
|
BMP (potassium) |
Hyperkalemia usually is present in ESRD patients due to reduced excretion of potassium. (1) Normal potassium: 3.5 – 5 mmol/L |
|
BMP (serum albumin) |
Can be low due to proteinuria or malnutrition (1) Normal serum albumin: 3.4 – 5.4 g/dL
|
|
BMP (Serum phosphate, 25-hydroxyvitamin D, alkaline phosphatase, and parathyroid hormone) |
Used to evaluate renal bone disease (1) Hyperphosphatemia Low 25-hydroxyvitamin D Elevated alkaline phosphatase High PTH levels |
|
Urinalysis |
Urine protein/creatine ratio to detect albuminuria (1) >30 mg albumin / g creatine = renal disease |
|
Urinalysis |
24-hr protein level (1) >3.5 g concerning for nephrotic proteinuria |
|
ABG |
Metabolic acidosis is associated with severe ESRD (1) Normal pH: 7.35 – 7.45 |
References
[1] Benjamin, O., & Lappin, S. L. (2022). End-stage Renal Disease. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK499861/
[2] Advanced Renal Education Program. (2020, March). Dialysis Disequilibrium Syndrome (DDS). https://advancedrenaleducation.com/wparep/article/disequilibrium-syndrome/
[3] Mistry, K. (2019). Dialysis disequilibrium syndrome prevention and management. International Journal of Nephrology and Renovascular Disease, 12, 69-77. https://doi.org/10.2147/ijnrd.s165925
[4] Patel, S., Jose, A., & Mohiuddin, S. S. (2022). Physiology, oxygen transport and carbon dioxide dissociation curve. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK539815/
[5] Arieff, A. I. (1994). Dialysis disequilibrium syndrome: Current concepts on pathogenesis and prevention. Kidney International, 45(3), 629-635. https://doi.org/10.1038/ki.1994.84
[6] Mayo Clinic. (2022, May 4). Hypoglycemia. https://www.mayoclinic.org/diseases-conditions/hypoglycemia/symptoms-causes/syc-20373685
[7] Kitabchi, A. E., Umpierrez, G. E., Miles, J. M., & Fisher, J. N. (2009). Hyperglycemic crises in adult patients with diabetes. Diabetes Care, 32(7), 1335-1343. https://doi.org/10.2337/dc09-9032
[8] Mayo Clinic. (2020, June 27). Hyperglycemia. https://www.mayoclinic.org/diseases-conditions/hyperglycemia/symptoms-causes/syc-20373631
[9] Freeman, W. D., & Aguilar, M. I. (2012). Intracranial hemorrhage: Diagnosis and management. Neurologic Clinics, 30(1), 211-240. https://doi.org/10.1016/j.ncl.2011.09.002
[10] Khaku, A. S., Tadi, P., & Gunn, A. A. (2022). Cerebrovascular disease (nursing). StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK568674/
[11] Mount, H. R., & Boyle, S. D. (2017). Aseptic and bacterial meningitis: Evaluation, treatment, and prevention. American Family Physician, 96(5), 314-332. https://www.aafp.org/pubs/afp/issues/2017/0901/p314.html
[12] Outsource Strategies International. (2017, October 26). Encephalopathy – ICD 10 coding and documentation guidelines. https://www.outsourcestrategies.com/resources/encephalopathy-icd-10-coding-documentation-guidelines/
[13] National Institute of Neurological Disorders and Stroke. (2022, July 25). Encephalopathy. https://www.ninds.nih.gov/health-information/disorders/encephalopathy
[14] Adrogue, H. J., Tucker, B. M., & Madias, N. E. (2022). Diagnosis and management of hyponatremia: A review. JAMA, 328(3), 280-291. https://doi.org/10.1001/jama.2022.11176
[15] Holley, J. D. (2020, January 30). Acute complications during hemodialysis. UpToDate. https://www.uptodate.com/contents/acute-complications-during-hemodialysis#!#:~:text=Acute%20complications%20commonly%20occur%20during%20routine%20hemodialysis%20treatments.,5%20to%2015%20percent%20Headache%20%E2%80%93%205%20percent
[16] Polkinghorne, K. R., & Kerr, P. G. (2016, June 4). Acute complications during hemodialysis. Abdominal Key. https://abdominalkey.com/acute-complications-during-hemodialysis/
[17] Henrich, W. L., & Flythe, J. E. (2021, December 21). Intradialytic hypotension in an otherwise stable patient. UpToDate. https://www.uptodate.com/contents/intradialytic-hypotension-in-an-otherwise-stable-patient#!#:~:text=Intradialytic%20hypotension%20may%20reduce%20the%20efficacy%20of%20the,of%20intradialytic%20hypotension%20in%20an%20otherwise%20stable%20patient.
[18] Agarwal, R., Alborzi, P., Satyan, S., & Light, R. P. (2009). Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension, 53(3), 500-507. https://doi.org/10.1161/hypertensionaha.108.125674
[19] Advanced Renal Education Program. (2021, March). Acute hemolysis. https://advancedrenaleducation.com/wparep/article/hemolysis/
[20] Berns, J. S., & Qunibi, W. Y. (2022, January 18). Treatment of anemia in dialysis patients. UpToDate. https://www.bing.com/search?q=upto+date+treatment+of+anemia+in+dialysis+patients&cvid=ee6622d045e34fb5a3e830f3851c6eab&aqs=edge..69i57j0l8.7870j0j1&pglt=299&FORM=ANNTA1&PC=HCTS

Jonathan Mohnkern, BS, FP-C, CP-C, NRP, has worked in the EMS field for the past eight years with a background in rural, suburban, and urban settings. He currently operates as a 911 and transport paramedic in Upstate, NY. Jon holds a bachelor’s degree in biology and psychology, has experience in EMS management and tactical medicine, and is a current medical non-commissioned officer in the New York Army National Guard. Jon is currently in medical school as a first-year medical student and is passionate about critical care, pre-hospital medicine, and EMS education.
