Crashing the Vented Patient – A Critical Care Odyssey

The Case
Your team has been requested for an interfacility transfer. You find a 35-year-old female writhing on the stretcher, kicking her feet, hands restrained, and a wide-eyed ED technician attempting to hold her legs down. The patient is intubated, and the ventilator has a near-constant high-pressure alarm.
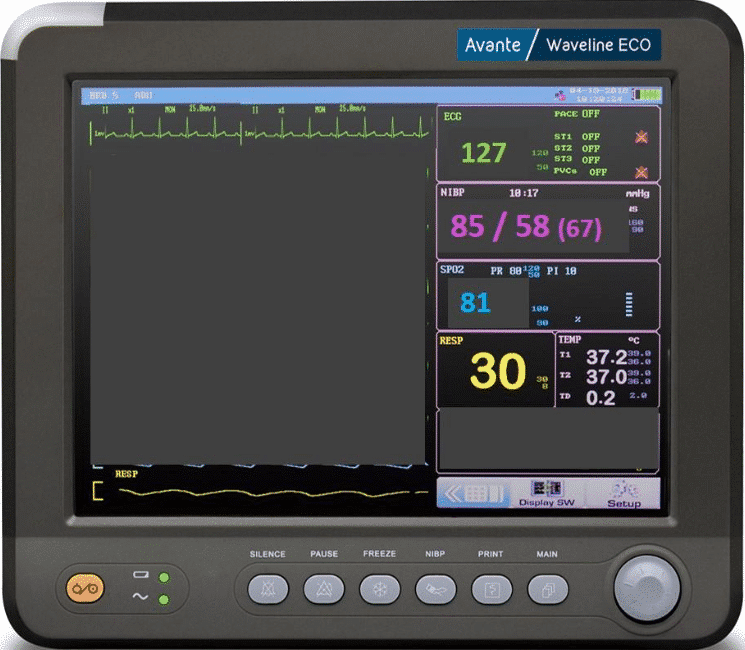
You notice the patient’s vitals and are quite concerned. The sending team steps in to provide a hand-off report:
“She was out drinking with friends last night and passed out in the bushes. They could not wake her up, so they called EMS and brought her here. She presented altered and drooly. Doc was worried about her airway, so he intubated her for airway protection. Her scans are all negative, and there are no physical signs of injury. Doc is sending her to University because she has been getting more hypotensive and hypoxic over the last five hours, and we cannot figure out why. It is also almost impossible to keep her sedated and from bucking the vent. Her tox screens are all negative, except for the booze, and her scans are all clean: no PEs, no internal bleeding…nothing. We started with levo and then added epi. Her propofol is still up because we cannot keep her sedated. This vent will not stop alarming!”
The chart does not reveal anything different from what the sending nurse explained. There is no known medical history, negative HCG, and no known medications. She is 5’8” inches tall and weighs 66kg.
The RT hands you a fresh blood gas:
pH: 7.12
PCo2PCO2: 67
PO2: 145
HCO3: 22
The sending facility ventilator has these values:
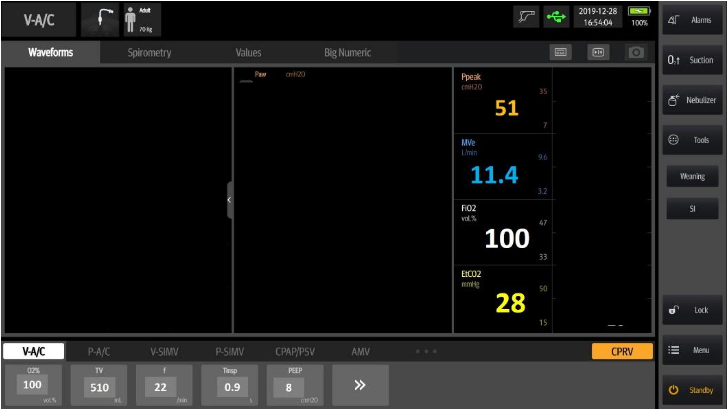
What is the problem? Why is the patient hypotensive, hypoxic, hypercapnic, and seemingly in shock? There are so many paths that a well-intended clinician could take to try to correct this. Paralysis? Perhaps. It will keep the patient from moving and more compliant with the ventilator, but is the dys-synchrony the problem or a symptom? Where is this respiratory acidosis coming from? Perhaps the most important question:
Why is this patient receiving 11 liters of minute volume?
Can the vent settings be the problem?
Crashing the Vent Patient: Ventilator-Induced Obstructive Shock
Over the last decade, ventilator management strategies have undergone many modifications. In some cases, have been completely revolutionized to focus on patient safety rather than the arbitrary normalization of numbers. Despite the evidence to support lung protective strategies, critical care transport teams often encounter patients exposed to inappropriate and/or inadequate mechanical ventilation through management strategies that not only neglect lung protection but may also contribute to the patient’s hemodynamic deterioration.
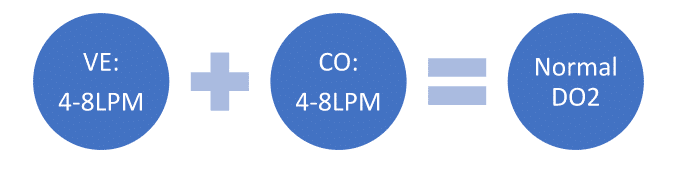
The heart and lungs do not operate in isolation from each other., In fact, they have a rather codependent relationship, and it is paramount to protect that relationship to maintain the needed oxygenation and ventilation to meet metabolic demands. At the most basic level, a person must bring in 4-8lpm of oxygen so that the heart can put out 4-8lpm of oxygenated blood., This facilitates normal oxygen delivery (assuming no oxygen-carrying capacity loss).
When there is a problem with minute ventilation or cardiac output, resuscitation must occur, sometimes leading to intubation and management with mechanical ventilation. For that resuscitation to be effective, it must be done safely. Unnecessarily high respiratory rates and tidal volumes (read: overventilation) can contribute to overall patient decompensation and exacerbation of metabolic derangement. Whereas it is widely understood that minute ventilation is the compensatory mechanism for metabolic acidosis, it is only that…compensation. It never corrects a metabolic acidosis, and often critical care teams find patients being dangerously over-ventilated with the justification that they need this dangerously high amount of minute ventilation to correct metabolic acidosis. This deeply flawed logic precipitates a host of untoward side effects, including ARDS, VILI, barotrauma, and biotrauma in the lungs.
Let’s break down each component of the problem
The Respiratory Component – Obstructive Physiology
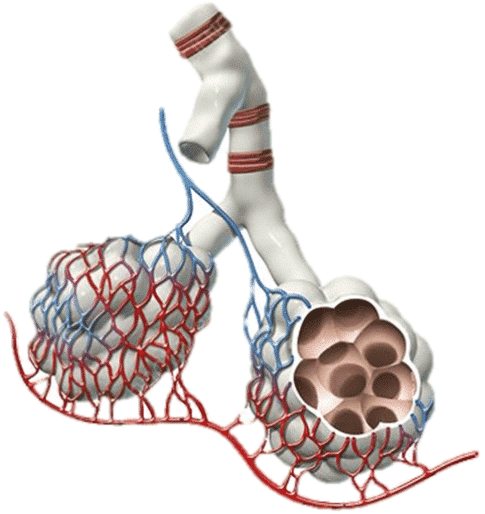
Overventilation to the point that it is dangerous for a patient is not all that dissimilar from the pathophysiology of obstructive lung diseases like COPD or asthma. Instead of a disease process that narrows the airways obstructing air movement, it is an iatrogenic time cycling problem where breath stacking and AutoPEEP occur that over distends the alveoli and lung parenchyma and compresses the surrounding pulmonary and thoracic vasculature. It is, in essence, an etiology of obstructive shock (not unlike a tension pneumothorax).
When the alveoli are over-distended, the pulmonary vasculature is compressed (a blanching type of compression), which increases pulmonary vascular resistance (PVR) and causes pulmonary hypertension. The compressed vasculature does not allow blood to flow freely to the pulmonary capillaries to exchange gas. This means there are areas of the lungs where the alveoli are being ventilated, but there is poor or absent blood flow. This is known as shunt physiology, and this specific type of shunt physiology is known as a ventilation/perfusion, or V/Q, mismatch.
Acid-Base Balance
AutoPEEP and breath stacking means that a patient is not fully exhaling the previous breath before the next one is delivered. Beyond the hyperinflation of the lungs, the problem here is that CO2 is being retained with each breath, and the obvious complication is that the pH begins to drop once the CO2 begins to climb out of the normal ranges. Respiratory acidosis is now complicating the situation. In cases with metabolic acidosis present, there is now a mixed disturbance that will be even more difficult to untangle for a transport team.
While the cardiovascular system is at risk of collapse just from the over-pressurization of the vasculature, causing the pump to fail, the patient has little physiologic response left to address respiratory acidosis. This acidosis further complicates the already tenuous cardiovascular situation by reducing the efficiency and function of the heart with reduced LV elastance, inotropy, and beta response.
The normal response would be adding vasopressors to support and improve cardiac output. This may have only a limited effect on temporizing hemodynamics. Provided that the patient’s pH is still within a range that the medications will not be rendered inert, the action of the vasopressors will increase the PVR and SVR, potentially worsening the already existing increased RV afterload. Critical care teams may continually escalate doses and add vasopressors to maintain a perfusing MAP.
Something else to consider in this situation is the current strategy for sedation and pain management. With this rising CO2 and lowering pH, the vent settings directly conflict with what the patient’s respiratory centers in the pons and medulla are likely screaming at the brain to do. The patient literally cannot respond to the commands received based on the chemical feedback they are receiving from the brainstem. Imagine how uncomfortable it must be to receive supra-normal minute ventilation, and on top of that, feel like you cannot breathe the way the brain is commanding you to. This is a perfect recipe for patient-ventilator diys-synchrony. This patient is likely going to present under-sedated with little attenuation of their pain, not because of “poor care,” but more from the fact that the pain and sedation strategy is not robust enough to overcome the patient’s intrinsic desire/need to breathe in response to their blood chemistry.
The Cardiac Component – Cor Pulmonale and Right Ventricular Failure
The negative effects of increased PVR and pulmonary hypertension greatly impact the patient’s ability to move blood through the lungs and into the left side of the heart. Increasing PVR and pulmonary hypertension increase right ventricular afterload, the pressure the right ventricle (RV) has to pump against to move blood into the lungs. The problem is that the RV is a low-pressure pump and can be quite fragile. It does not have the same musculature as the left ventricle (LV), and it does not take long before the increased afterload begins to overwhelm the RV and cause it to dilate and fail to function. Once the RV becomes dilated, it essentially acts as a conduit through which de-oxygenated blood flows passively into the lungs (completely dependent on the contractile force of the LV). Doing so with dramatically reduced output.
The overdistention of the lungs leads to a secondary effect of compressing the thoracic vasculature, most notably the inferior vena cava.
This is the beginning of a dangerous downward spiral. Not only is there a V/Q mismatch present, but there is an overall reduction in cardiac output secondary to the poor function of the RV. Hypoxia abounds in this situation and serves to exacerbate these already dangerous conditions.
Fixing the Overventilated Patient – An Approach
How do critical care transport teams handle these types of issues? Many elements color the context of these situations, and it should be understood that there is absolutely no “one-size-fits-all” approach.
There are high-pressure alarms, patient/ventilator dyis-synchrony, and tenuous vitals in this situation. One may feel compelled to take the patient off the ventilator and bag the patient with a BVM. This should be viewed as a temporary, last-resort measure as it is a suboptimal way to deliver appropriate minute volume to the patient.
SKiPs – Start Here
I called this the SKiIPS checklist because, in my experience, most novice transport clinicians or teams in a hurry will “skip” this checklist and instead start twisting knobs on the vent to “fix” things. However, neglecting these items could mean that the clinician misses an easily controllable variable or continues to make adjustments to ventilation with a patient who does not have their pain well controlled and may not be appropriately sedated. In this situation, removing as many influences on your feedback devices as possible is important so that the true problem can be isolated. A good pain control/sedation strategy can take the patient/vent dyis-synchrony out of the equation and assist with clearing up the clinical picture.
S – – Suction the tube and confirm the airway is in the right place.
Ki – Evaluate for Kinks in the circuit, feedback lines, or the ET tube
P – Is Pain adequately controlled?
S – Is Sedation adequate?
Do Your Own Work
Once the patient factors have been isolated and dealt with, the team can move on to more precise troubleshooting. Evaluate what the patient is receiving from the sending facility ventilator, then perform your calculations as to what the patient should be receiving based on their height, IBW, and underlying pathology.
- Calculate the appropriate MV.
- It should be somewhere between 4-8lpm
- If there is a known metabolic acidosis, consult with your team’s physician and develop a plan.
- Calculate the appropriate tidal volume based on the patient’s IBW.
- It should be between 6-8cc/kg IBW
- Calculate the appropriate respiratory rate.
- Divide MV/vTVt = RR
A metabolic disorder can make this somewhat more complicated. These patients need more minute volume to help compensate, but clinicians must be intentional with their actions. The issue may not be the calculated MV, or the vTVt may not be terribly inappropriate, but an I:E ratio issue should be evaluated. Regardless of the underlying metabolic issues and targeted minute volume needs, clinicians must ensure enough time to cycle full inspiratory and expiratory phases. By not allowing time for a full respiratory cycle, the patient is at risk for the issues associated with breath stacking and autoPEEP we discussed above.
Next Steps
At this point, the patient should have their pain well controlled, be adequately sedated, have no tube DOPE issues, and receive physiologically appropriate ventilator settings. Periodic evaluation of blood gas samples will be needed to drive the rest of the resuscitation strategy. Initiation or escalation of vasopressor therapy may be needed, or they could even be de-escalated now that the source of hemodynamic instability has been discovered and corrected.
Conclusion
Occasionally, critical care transport teams may be presented with a patient in profound shock that does not appear as obvious as other etiologies. In this case, the most important thing to do is find the source and then go back to the basics to correct the problem.

Cody Winniford, BA, EMT-P, CCP-C, FP-C
Cody has been in EMS for 18 years, serving in both military and civilian settings. His professional experiences include serving as an educator for initial paramedic instruction programs, clinical manager, EMS operations manager, and EMS Operations Director. He is currently serving as a flight paramedic with PHI Air Medical and volunteers for the education committee for the ICAPP.
