The Intricacies of Pre-Hospital Trauma Triage: a Look into Special Considerations

Introduction
A crew responds to a motor vehicle collision involving a passenger vehicle into a tree. The scene size-up illustrates moderate front-end damage and airbag deployment. Further analysis of the car shows no apparent signs of passenger compartment intrusion, steering wheel deformity, dash deformity, evidence of rollover, or window spidering. Your patient (75 male), a single occupant of the vehicle, is walking around the scene and is “a little shaken up but overall okay,” by self-report. He complains of mild nausea but attributes that to anxiety. The patient admits he was fully restrained and traveling approximately 40 mph around a curve when he lost control of the vehicle, causing it to move off the road and into a nearby tree. There is no evidence of acute medical conditions that led to a loss of consciousness or deviation in alertness. The patient is moved into the ambulance for further assessment by the paramedic.
Trauma Center Criteria
Patients from motor vehicle collisions, assaults, and ground-level falls frequently refuse transport to the hospital due to the absence of obvious injuries. EMS providers are experiencing traumatic calls daily and are expected to make sound clinical decisions regarding appropriate disposition. A critical piece in managing trauma patients is determining the appropriate destination, specifically whether to transport them to a trauma center. The National Guideline for the Field Triage of Injured Patients guides trauma center criteria while considering four primary categories: vital signs & mental status, injury patterns, mechanism of injury, and EMS judgment. This discussion will focus on the latter, specifically on patients who are at high susceptibility to pathological bleeding.
Hemostasis
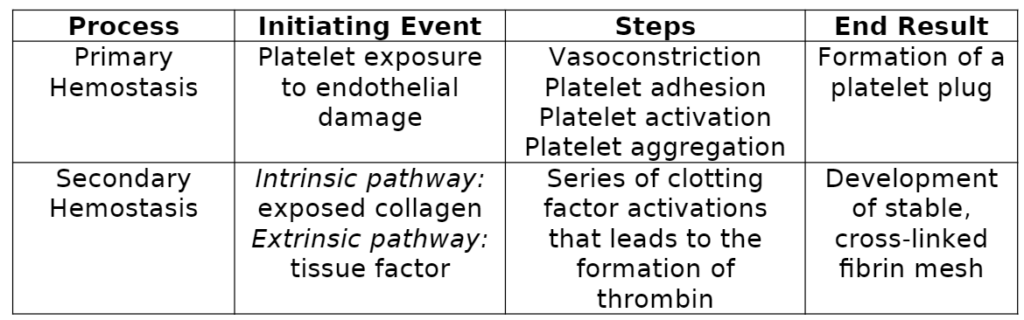
Before discussing special considerations about bleeding, we must first review hemostasis. Hemostasis refers to the physiological steps that your body takes to stop bleeding. There are two major types: Primary Hemostasis: Under normal function, platelets circulate throughout your bloodstream and are “primed” to respond to endothelial injury. Vascular injury exposes sub-endothelial structures to which platelets rapidly adhere (1,2). Platelets activate fibrinogen receptors through intracellular signaling pathways, leading to aggregation (1,2). In addition, activated platelets release cellular contents (dense and α-granules) that activate nearby platelets to assist in injury response. Activated platelets express phospholipids that lead to the development of localized coagulation (1). Overall, the function of primary hemostasis is to form a platelet plug to minimize the extent of blood loss. Secondary Hemostasis: Secondary hemostasis produces a stable fibrin mesh through a complex pathway known as the coagulation cascade. The coagulation cascade comprises an intrinsic and extrinsic pathway, both leading to the activation of clotting factor X, which signifies the beginning of the common pathway (shared between intrinsic and extrinsic). The intrinsic pathway is stimulated by exposed collagen from damaged vascular endothelium (3). On the other hand, the extrinsic pathway is initiated by exposure to tissue factor, which is released from damaged vasculature (3). The common pathway produces thrombin, a critical enzyme in the cascade with various functions. For example, thrombin activates platelets, cleaves fibrinogen to produce fibrin, and activates clotting factor XIII, which binds with calcium to produce stable fibrin crosslinks. The intended consequence of secondary hemostasis is to create a stable clot that prevents extensive blood loss from vascular injury (4).
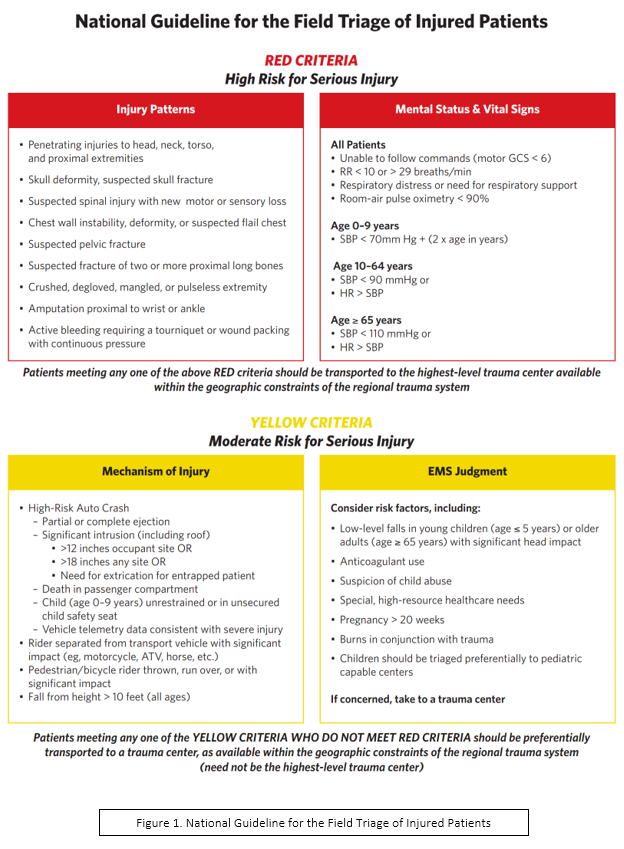
What patients fall under “EMS Judgment” in the National Guideline for the Field Triage of Injured Patients?
Medications
Anticoagulants are easily one of the clinicians’ first considerations when evaluating a trauma patient. These medications are primarily administered to reduce the risk of thromboembolism. However, one of the concerning effects of these medications is that they increase the extent of bleeding by interfering with the coagulation cascade we just reviewed.
Vitamin K Antagonists: Vitamin K is a co-factor of the γ-glutamyl carboxylase enzyme that resides within the endoplasmic reticulum of cells. This enzyme converts specific amino acid glutamate residues to γ-carboxyglutamate in various vitamin-K-dependent proteins (5). This process is critical in producing multiple coagulation factors (II, VII, IX, & X). Warfarin (Coumadin) is a well-known vitamin-K antagonist (VKA) that blocks the vitamin K-epoxide reductase (6). Ultimately, this inhibits the activation of vitamin K-dependent coagulation factors. Interestingly, Warfarin is initially prothrombin as it blocks the effects of Protein C and S (natural anticoagulants) before it inhibits coagulation factors (6). Alternative anticoagulants have been developed and utilized due to the narrow therapeutic index and frequent requirement of INR analysis while taking Warfarin (7).
Direct Factor Xa Inhibitors: A class of medications including apixaban (Eliquis), rivaroxaban (Xarelto), and edoxaban (Savaysa). As their name implies, these anticoagulants reversibly, directly, and selectively inhibit clotting factor Xa (7,8,9). Therefore, they decrease thrombin production and clot formation. In addition, they indirectly reduce platelet aggregation, usually stimulated by thrombin (7,8,9).
Direct Thrombin Inhibitors: Dabigatran (Pradaxa) is a common direct thrombin inhibitor that binds specifically to the thrombin’s active site (7). Like the direct factor Xa inhibitors, dabigatran blocks thrombin-mediated events such as the degradation of fibrinogen, stimulation of various coagulation factors, and platelet aggregation (9).
Aspirin: Acetylsalicylic acid (ASA) belongs to the non-steroid anti-inflammatory drug class. Many patients are taking low-dose ASA to reduce the risk of cardiovascular disease. In theory, low-dose ASA irreversibly inhibits cyclooxygenase-1 (COX-1) activity, which generally plays a role in prostaglandin H2 synthesis (10,11). This prostaglandin is a precursor to thromboxane A2, a hormone critical in platelet aggregation. Ultimately, ASA reduces platelet aggregation through the downregulation of dense granule release, customarily utilized to activate other platelets (10).
EMS providers must hold a high index of suspicion for significant hemorrhage in all patients taking anticoagulants or antiplatelets, even in cases of lower mechanism of injury. There is some variation in studies about what anticoagulants or antiplatelets pose the most risk, but ultimately most evidence supports that this patient population is more susceptible to hemorrhage (12,13,14). Most current evidence focuses on the increased prevalence of intracranial hemorrhage in anticoagulated patients (14). Anticoagulants also increase the risk of bleeding in the thoracic, abdominal, and retroperitoneal cavities, among other locations (15). Furthermore, anticoagulant use in the context of trauma is related to an increased incidence of death, worse clinical outcomes, and a higher likelihood of being discharged to an intensive facility even after controlling for age (16).
Bleeding Disorders
There are a variety of medical conditions that increase a patient’s susceptibility to hemorrhage and are essential to consider during an assessment.
Hemophilia: This disease is a deficiency in one of the clotting factors we discussed previously. For example, Hemophilia A is the most common, related to a reduction in functional clotting factor VIII. This condition is either congenital through vertical transmission in an X-linked recessive manner or acquired as a secondary effect of medical conditions (17). Some examples associated with acquired hemophilia may include systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, hematological malignancies, postpartum hemorrhage, hepatitis, or bacterial infections treated with penicillin (17,18). Patients with hemophilia exhibit a wide range of severity depending on the extent of deficiency in a particular clotting factor, prescribed medications, and other underlying medical conditions. Regardless, all patients with hemophilia or unexplained hemorrhage require a high index of suspicion while performing a trauma assessment.
Von Willebrand Disease (VWD): This condition is the most common bleeding disorder and affects up to 1% of the United States population (19). VWD has various subsets with lower levels or non-functional von Willebrand factor (vWF). Earlier, we discussed primary hemostasis and its function in creating a platelet plug to reduce initial hemorrhage. Von Willebrand factor is a glycoprotein that plays a crucial role in primary hemostasis, particularly in platelet adhesion (20). Furthermore, vWF is also a chaperone for coagulation factor VIII, which degrades rapidly in the blood if it is unbound (20). Therefore, patients with lower functional levels of this protein will have impaired hemostasis and an increased risk of severe hemorrhage (19).
Vitamin K Deficiency: As discussed previously, the involvement of vitamin K is imperative in the coagulation cascade. It is a co-factor to an enzyme that produces functional coagulation factors II, VII, IX, & X. This condition commonly refers to the neonatal period as this patient population is most susceptible due to insufficient vitamin K placental transfer and deficient levels in breast milk (21). It usually presents as gastrointestinal, umbilical, or intracranial hemorrhage. The severity of this condition led to the creation of vitamin K prophylaxis treatments (21). While substantially rare due to the small daily requirement of vitamin K, acquired vitamin K deficiencies may occur in adults with severe malabsorption, recent major surgery, long-term parenteral nutrition, or broad-spectrum antibiotics (22). Furthermore, it is also possible for individuals to develop acquired vitamin K deficiency through bulimia or anticoagulant use. Like other bleeding disorders, a lack of vitamin K will lead to a less-than-optimal coagulation cascade, delayed clotting, and a higher risk of severe hemorrhage.
Bleeding disorders are rare and infrequently seen in most patient populations; however, they present a severe risk of hemorrhage in cases of low mechanism of injury. It is essential to acknowledge that not all patients have been diagnosed with a definitive cause of inadequate clotting, which is further complicated due to the acquired nature of some of these disorders. Therefore, it is helpful to know some of the “red flags” that patients disclose that may indicate the need to have a coagulation panel performed.
Possible signs of undiagnosed bleeding disorders
- Epistaxis more than five times per year that lasts over 10 minutes
- Prolonged bleeding from simple abrasions/lacerations (> 10 minutes)
- Easy bruising from minimal impact
- Anemia treatment
- Significant bleeding after surgeries or dental procedures
- Family history of bleeding disorders
- Heavy menstrual bleeding
National Hemophilia Foundation (19)
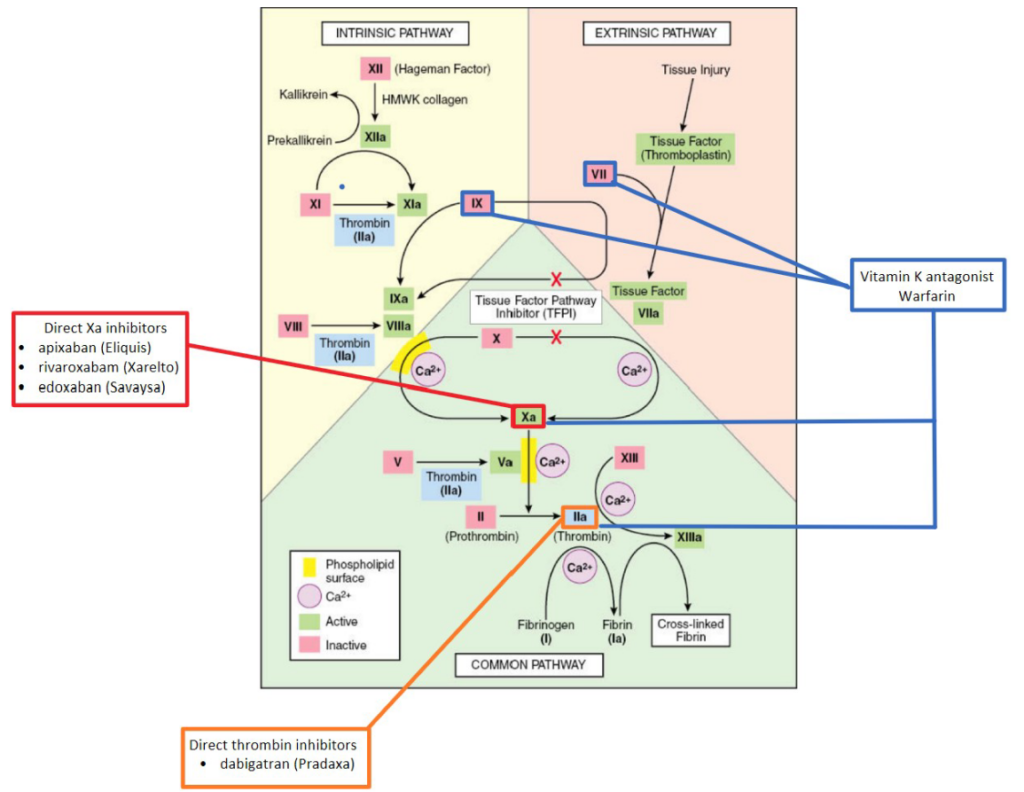
Figure 2. Coagulation Cascade
Coexisting Medical Conditions
Increasing Age: Various physiological changes occur with aging, increasing the risk of pathological clotting in this population. In addition to the increased development of cardiovascular risk factors such as CAD, diabetes, and hypertension, elderly patients also develop increasing levels of coagulation proteins (23). Interestingly, this prothrombic state does not protect elderly patients in traumatic incidents since they rapidly transition to a hypercoagulable state and suffer more severe bleeding consequences than their younger counterparts (24). Physiological changes, concomitant medical conditions, and polypharmacy complicate the diagnosis of internal hemorrhage or early hemodynamic instability based on vital signs (25). As a result, it is safe to say that elderly trauma patients require a higher index of suspicion and a more liberal designation to a trauma center.
Chronic Kidney Disease: Like aging, CKD has been related to increased pathological thrombosis and risk of bleeding (26). This relationship is particularly true in significant decreases in glomerular filtration rate (GFR) or albuminuria (26). It is believed that CKD leads to increased bleeding due to the use of anticoagulants, intrinsic deficiency in platelets, and endothelial dysfunction (26). Studies have shown the risk of thromboembolism and Figure 2. Coagulation Cascade bleeding is even higher in patients with ESRD and cardiovascular conditions such as atrial fibrillation (27).
Hepatic Disease: The liver produces coagulation factors, natural anticoagulants, proteins involved in fibrinolysis, and thrombopoietin (28). Further, thrombocytopenia is common due to reduced thrombopoietin production and splenic consumption. As a result, liver disease significantly alters hemostasis and increases the risk of bleeding and thromboembolism. To further complicate the situation, standard coagulation tests (PT, INR) are not an accurate assessment in these patients as they have developed a hemostatic balance different from healthy patients (28,29). Due to this hemostatic balance, not all patients with liver disease are at increased risk of bleeding. Many of these patients require a case-by-case assessment and treatment plan, frequently with the consultation of a specialist (29,30). The complexity of these patients is challenging to evaluate in the pre-hospital setting. It may be best to transport this patient to a trauma center since we know disrupted hemostasis is plausible.
Thrombocytopenia: This condition refers to a low platelet count. Normal blood platelet levels usually range between 150,000 and 400,000/μL. Individuals with thrombocytopenia are at higher risk for prolonged and severe bleeding (31). There are numerous causes for this condition. First, individuals with primary immune thrombocytopenia develop antibodies against platelets that ultimately lead to their destruction (31). Various drugs such as heparin, quinine, antibiotics, & NSAIDs are all documented as causing thrombocytopenia. Concomitant medical conditions such as malignancies, active or chronic infectious diseases (HIV, hepatitis C, EBV, H. pylori, etc.), and sepsis are other frequent causes of thrombocytopenia. Lastly, chronic alcohol abuse and liver disease are related to hypersplenism and increased consumption of platelets (31). In the pre-hospital setting, we do not always have access to recent bloodwork or knowledge of all these conditions. As a result, it may be helpful to consider the increased risk of bleeding due to thrombocytopenia in patients with a history of active malignancy, ongoing infectious disease, or liver disease.
Pregnancy: Pregnancy presents unique complications that must be considered even after seemingly minor incidents. Not only do we have to think about the health of the mother, but also of the fetus. Trauma has become more common during pregnancy and is now considered the number one cause of non-obstetrical maternal death (32). Increased incidences of falls, domestic violence, and improper seat belt use are a few examples of pregnancy-related trauma (32). Specific injury patterns are particularly worrisome during pregnancy. For instance, pregnancy increases the risk of hemorrhage from pelvic fractures due to dilated vasculature. Pelvic fractures are frequently related to fetal death from direct fetal injury, placental abruption, or maternal shock (32). Placental abruption is the separation of the placenta from the uterine wall and can occur during minor trauma. The classic triad includes vaginal bleeding, abdominal pain, and uterine irritability; however, it is essential to acknowledge that not all patients will present with this triad. Some may show little to no visible bleeding due to concealment (33). In severe cases, placental abruption may lead to disseminated intravascular coagulation and fetal distress. Uterine rupture is typically related to significant forces and presents with abdominal pain, distention, palpable fetal parts, and shock. Pain may not be a reliable indicator of this condition, however, due to reduced peritoneal sensitivity in the third trimester (32).
To add another layer, physiological changes during pregnancy complicate our routine assessment. For example, “standard” signs of shock, including tachycardia and hypotension (excluding 3rd trimester), may be typical for pregnant patients. Further, increased plasma volume may lead to a delay in the recognition of shock (32). Ultimately, EMS providers should maintain a high index of suspicion in these patients as assessment may be convoluted due to various physiological changes.
Placental Abruption (33)
Diagnostic Tools: clinical presentation, ultrasound, coagulation testing, & fetal heart monitoring (note: normal ultrasound findings do not rule out placental abruption)
Treatment
- Prompt cesarean delivery – maternal instability, non-reassuring fetal heart pattern, & term pregnancy (> 37 weeks)
- Vaginal delivery – mother hemodynamically stable, reassuring fetal heart pattern, & term pregnancy
- Hospitalization & observation – non-life-threatening bleeding, reassuring fetal heart pattern, & pre-term (< 37 weeks)
- Corticosteroid consideration to accelerate fetal lung maturity if gestational age 34-36 weeks, no previous corticosteroid administration, no contraindications, & risk of late pre-term is high
The National Guideline for the Field Triage of Injured Patients is an excellent resource to assist pre-hospital providers in determining the disposition of trauma patients. However, the EMS judgment section remains ambiguous, and for a good reason. One, not all patients read the textbook – patients will have different presentations and still may be best managed at a trauma center. Next, the choices you make daily in the field consider an endless number of factors: resources available, patient stability, location of the trauma center, transport vehicle, etc. Lastly, EMS judgment allows pre-hospital providers to follow their instinct and make choices that they feel are in their patient’s best interest. This article aims to help sift through some of the nuances of bleeding considerations and assist in breaking down the importance of appropriate trauma designation.
References
1) Berndt MC, Metharom P, Andrews RK. Primary hemostasis: Newer insights. Haemophilia. 2014;20(4):15-22. https://doi.org/10.1111/hae.12427
2) Holinstat M. Normal platelet function. Cancer Metastasis Reviews. 2017;36(2):195-198. https://doi.org/10.1007/s10555-017-9677-x
3) Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Critical Reviews in Biochemistry and Molecular Biology. 2015;50(4): 326-336. https://doi.org/10.3109/10409238.2015.1050550
4) Cleveland Clinic medical professionals. Hemostasis. Clevelandclinic.org. Published December 8, 2021. Accessed September 28, 2022. https://my.clevelandclinic.org/health/symptoms/21999-hemostasis
5) Hao Z, Jin DY, Chen X, Schurgers LJ, Stafford DW, Tie JK. γ-glutamyl carboxylase mutations differentially affect the biological function of vitamin K-dependent proteins. Blood. 2021;137(4):533-543. https://doi.org/10.1182/blood.2020006329
6) Harter K, Levine M, Henderson SO. Anticoagulation drug therapy: A review. Western Journal of Medicine. 2015;16(1):11-17. https://doi.org/10.5811/westjem.2014.12.22933
7) Wonkyung B, Garonzik S, Boyd RA, Frost CE. Apixaban: A clinical pharmacokinetic and pharmacodynamic review. Clinical Pharmacokinetics. 2019;58(10): 1265-1279. https://doi.org/10.1007/s40262-019-00775-z
8) Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: Pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thrombosis Journal. 2013;11(1):10. https://doi.org/10.1186/1477-9560-11-10
9) Sunkara T, Ofori E, Zarubin V, Caughey ME, Gaduputi V, Reddy M. Perioperative management of direct oral anticoagulants (DOACs): A systematic review. Health Services Insights. 2016;9(1):25-36. https://doi.org/10.4137/hsi.s40701
10) Mekaj YH, Daci FT, Mekaj AY. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Therapeutics and Clinical Risk Management. 2015;11:1449-1456. https://doi.org/10.2147/TCRM.S92222
11) Abramsom SB, Furst DE, Seo P. Aspirin: Mechanism of action, major toxicities, and use in rheumatic diseases. UpToDate.com. Updated April 21, 2021. Accessed October 12, 2022. https://www.uptodate.com/contents/aspirin-mechanism-of-action-major-toxicities-and-use-in-rheumatic-diseases#
12) Ang D, Kurek S, McKenney M, Norwood S, Kimbrell B, Barquist E, Liu H, O’Dell A, Ziglar M, Hurst J. Outcomes of geriatric trauma patients on preinjury anticoagulation: A multicenter study. The American Surgeon. 2017;1(83):527-535. https://doi.org/10.1177/000313481708300614
13) Williams TM, Sadjadi J, Harken A, Victorino GP. The necessity to assess anticoagulation status in elderly injured patients. The Journal of Trauma: Injury, Infection, and Critical Care. 2008;65(4):772-777. https://doi.org/10.1097/ta.0b013e3181877ff7
14) Hecht JP, LaDuke ZJ, Cain-Nielsen AH, Hemmila MR, Wahl WL. Effect of preinjury oral anticoagulants on outcomes following traumatic brain injury from falls in older adults. Pharmacotherapy. 2020;40(7):604-613. https://doi.org/10.1002/phar.2435
15) Ohmori T, Kitamura T, Onishi H, Ishihara J, Nojima T, Yamamoto K. Effect of pre-injury anticoagulant and antiplatelet agents on blood loss in elderly patients with severe trauma. Acute Medicine and Surgery. 2015;3(2):114-119. https://doi.org/10.1002/ams2.152
16) Nguyen RK, Rizor JH, Damiani MP, Powers AJ, Fagnani JT, Monie DL, Cooper SS, Griffiths AD, Hellenthal NJ. The impact of anticoagulation on trauma outcomes: An national trauma data bank study. The American Surgeon. 2020;86(7). https://doi.org/10.1177/0003134820934419
17) National Heart, Lung, and Blood Institute. Bleeding disorders. NIH.gov. Updated March 24, 2022. Accessed October 17, 2022. https://www.nhlbi.nih.gov/health/bleeding-disorders
18) Yousphi AS, Bakhtiar A, Cheema MA, Nasim S, Ullah W. Acquired hemophilia A: A rare but potentially fatal bleeding disorder. Cureus. 2019;11(8). https://doi.org/10.7759/cureus.5442
19) National Hemophilia Foundation. Von Willebrand Disease. Accessed October 17, 2022. https://www.hemophilia.org/bleeding-disorders-a-z/types/von-willebrand-disease
20) Kiotptsi K, Reinhardt C. Physiological Roles of the von Willebrand Factor – Factor VIII Interaction. Sub-cellular Biochemistry. 2020;94:437-464. https://doi.org/10.1007/978-3-030-41769-7_18
21) Sankar MJ, Chandrasekaran A, Kumar P, Thukral A, Agarwal R, Paul VK. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: A systematic review. Journal of Perinatology. 2016;36(1):29-35. https://doi.org/10.1038/jp.2016.30
22) Zekavat OR, Fathpour G, Haghpanah S, Dehghani SJ, Zekavat M, Shakibazad N. Acquired vitamin K deficiency as unusual cause of bleeding tendency in adults: A case report of a nonhospitalized student presenting with severe menorrhagia. Case Reports in Obstetrics and Gynecology. 2017. https://doi.org/10.1155/2017/4239148
23) Mari D, Ogliari G, Castalde D, Vitale G, Bollini EM, Lio D. Hemostasis and aging. Immunity & Ageing. 2008;5(12). https://doi.org/10.1186/1742-4933-5-12
24) Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. Journal of Thrombosis and Thrombolysis. 2013;35(3):312-319. https://doi.org/10.1007/s11239-013-0899-7
25) Ohmori T, Kitamura T, Tanaka K, Saisaka Y, Ishihara J, Onishi H, Nojima T, Yamamoto K, Matsumoto T, Tokioka T. Bleeding sites in elderly trauma patients who require massive transfusion: A comparison with younger patients. The American Journal of Emergency Medicine. 2016;34(2):123-127. https://doi.org/10.1016/j.ajem.2015.09.047
26) Ocak G, Rookmaaker MB, Algra A, Borst GJ, Doevendans PA, Kappelle LJ, Verhaar MC, Visseren FL. Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: A cohort study. Journal of Thrombosis and Haemostasis. 2018;16(1):65-73. https://doi.org/10.1111/jth.13904
27) Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. The New England Journal of Medicine. 2012;367(7):625-635. https://doi.org/10.1056/nejmoa1105594
28) Flores B, Trivedi HD, Robson SC, Bonder A. Hemostasis, bleeding, and thrombosis in liver disease. Journal of Translational Science. 2017;3(3). https://doi.org/10.15761/jts.1000182
29) Armando T. Hemostasis abnormalities in cirrhosis. Current Opinion in Hematology. 2015;22(5):406-412. https://doi.org/10.1097/moh.0000000000000164
30) Northup PG, Lisman T, Roberts LN. Treatment of bleeding in patients with liver disease. The Journal of Thrombosis and Haemostasis. 2021;19(7):1644-1652. https://doi.org/10.1111/jth.15364
31) Jinna S, Khandhar PB. Thrombocytopenia. StatPearls Publishing; 2022. Accessed October 24, 2022. https://www.ncbi.nlm.nih.gov/books/NBK542208/
32) Krywko DM, Toy FK, Mahan ME, Kiel J. Pregnancy trauma. StatPearls Publishing; 2022. Accessed November 14, 2022. https://pubmed.ncbi.nlm.nih.gov/28613676/
33) Dulay AT. MSD Manual. Placental Abruption. Updated October, 2022. Accessed November 15, 2022. https://www.msdmanuals.com/professional/gynecology-and-obstetrics/abnormalities-of-pregnancy/placental-abruption-abruptio-placentae
34) Suchecki G, Tilden H, Roloff K, Chandwani D, Neeki M. Management of traumatic uterine rupture in blunt abdominal trauma: A case report and literature review. 2020;1(12). https://doi.org/10.7759/cureus.8396

Jonathan Mohnkern, BS, FP-C, CP-C, NRP, has worked in the EMS field for the past eight years with a background in rural, suburban, and urban settings. He currently operates as a 911 and transport paramedic in Upstate NY. Jon holds a bachelor’s degree in biology and psychology, has experience in EMS management and tactical medicine, and is a current medical non-commissioned officer in the New York Army National Guard. Jon is currently in medical school as a first-year medical student and is passionate about critical care, pre-hospital medicine, and EMS education.
